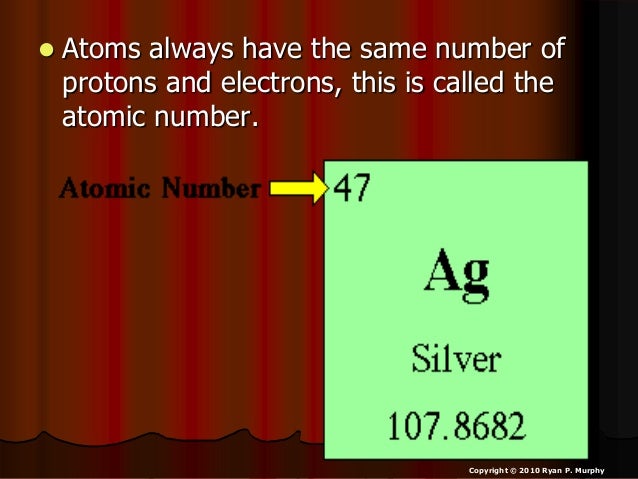
- How Many Protons Are In Silver
- Silver Atom With A Mass Number Of 109
- Silver-108 Mass Number
- Ag Atomic Mass
- Number Of Neutrons In Silver
Molecular mass (molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12) Molar mass (molar weight) is the mass of one mole of a substance and is expressed in g/mol. Weights of atoms and isotopes are from NIST article. Atomic Number Mass Number Number of protons Number of neutrons Number of electrons Symbol of Element 9 10 14 15 47 22 55 25 Br 8 16 47 61 16 16 Pb 2. What is the difference between mass number and atomic number 3. The atomic number of a Silver atom (Ag)=47 Therefore the number of protons in the nucleus =47 The mass number of Ag= 107g/mol 107 g / m o l Become a member and unlock all Study Answers.
Silver is extracted from the anode waste sludges of electrolytic copper-refining. Isotopes: Silver has 35 isotopes whose half-lives are known, with mass numbers 94 to 128. Naturally occurring silver is a mixture of its two stable isotopes, 107 Ag and 109 Ag with natural abundances of 51.8% and 48.2% respectively. Silver, chemical element of atomic number 47, a white lustrous metal valued for its decorative beauty and electrical conductivity. Silver’s physical and chemical properties are intermediate between those of copper and gold. It is located in Group 11 of the periodic table.
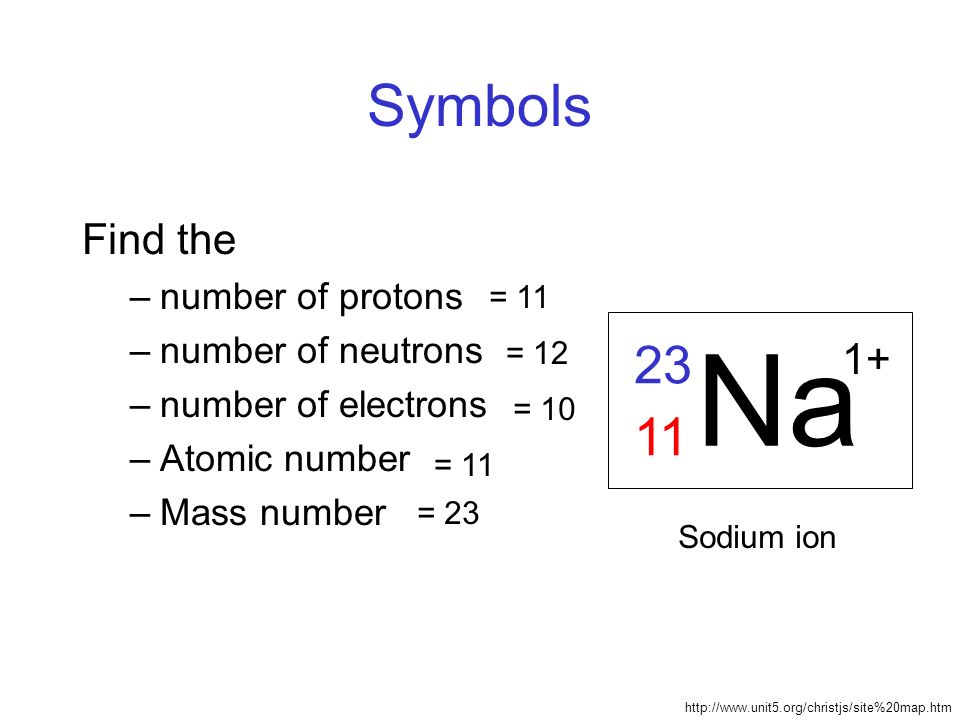
The mass number (symbol A, from the German word Atomgewicht [atomic weight]),[1] also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approximately equal to the atomic (also known as isotopic) mass of the atom expressed in atomic mass units. Since protons and neutrons are both baryons, the mass number A is identical with the baryon numberB of the nucleus (and also of the whole atom or ion). The mass number is different for each different isotope of a chemical element. Hence, the difference between the mass number and the atomic numberZ gives the number of neutrons (N) in a given nucleus: N = A − Z.[2]
The mass number is written either after the element name or as a superscript to the left of an element's symbol. For example, the most common isotope of carbon is carbon-12, or 12
C
, which has 6 protons and 6 neutrons. The full isotope symbol would also have the atomic number (Z) as a subscript to the left of the element symbol directly below the mass number: 12
6C
.[3]
Mass number changes in radioactive decay[edit]
Different types of radioactive decay are characterized by their changes in mass number as well as atomic number, according to the radioactive displacement law of Fajans and Soddy. For example, uranium-238 usually decays by alpha decay, where the nucleus loses two neutrons and two protons in the form of an alpha particle. Thus the atomic number and the number of neutrons each decrease by 2 (Z: 92 → 90, N: 146 → 144), so that the mass number decreases by 4 (A = 238 → 234); the result is an atom of thorium-234 and an alpha particle (4
2He2+
):[4]
| 238 92U | → | 234 90Th | + | 4 2He2+ |
On the other hand, carbon-14 decays by beta decay, whereby one neutron is transmuted into a proton with the emission of an electron and an antineutrino. Thus the atomic number increases by 1 (Z: 6 → 7) and the mass number remains the same (A = 14), while the number of neutrons decreases by 1 (N: 8 → 7).[5] The resulting atom is nitrogen-14, with seven protons and seven neutrons:
| 14 6C | → | 14 7N | + | e− | + | ν e |
Beta decay is possible because different isobars[6] have mass differences on the order of a few electron masses. If possible, a nuclide will undergo beta decay to an adjacent isobar with lower mass. In the absence of other decay modes, a cascade of beta decays terminates at the isobar with the lowest atomic mass.
Another type of radioactive decay without change in mass number is emission of a gamma ray from a nuclear isomer or metastable excited state of an atomic nucleus. Since all the protons and neutrons remain in the nucleus unchanged in this process, the mass number is also unchanged.
Mass number and isotopic mass[edit]
The mass number gives an estimate of the isotopic mass measured in atomic mass units (u). For 12C, the isotopic mass is exactly 12, since the atomic mass unit is defined as 1/12 of the mass of 12C. For other isotopes, the isotopic mass is usually within 0.1 u of the mass number. For example, 35Cl (17 protons and 18 neutrons) has a mass number of 35 and an isotopic mass of 34.96885.[7] The difference of the actual isotopic mass minus the mass number of an atom is known as the mass excess,[8] which for 35Cl is –0.03115. Mass excess should not be confused with mass defect which is the difference between the mass of an atom and its constituent particles (namely protons, neutrons and electrons).
There are two reasons for mass excess:
- The neutron is slightly heavier than the proton. This increases the mass of nuclei with more neutrons than protons relative to the atomic mass unit scale based on 12C with equal numbers of protons and neutrons.
- Nuclear binding energy varies between nuclei. A nucleus with greater binding energy has a lower total energy, and therefore a lower mass according to Einstein's mass–energy equivalence relation E = mc2. For 35Cl, the isotopic mass is less than 35, so this must be the dominant factor.
Relative atomic mass of an element[edit]
The mass number should also not be confused with the standard atomic weight (also called atomic weight) of an element, which is the ratio of the average atomic mass of the different isotopes of that element (weighted by abundance) to the unified atomic mass unit.[9] The atomic weight is an actual mass (made relative, i.e., a ratio), while the mass number is a counted number (and so an integer).
This weighted average can be quite different from the near-integer values for individual isotopic masses. For instance, there are two main isotopes of chlorine: chlorine-35 and chlorine-37. In any given sample of chlorine that has not been subjected to mass separation there will be roughly 75% of chlorine atoms which are chlorine-35 and only 25% of chlorine atoms which are chlorine-37. This gives chlorine a relative atomic mass of 35.5 (actually 35.4527 g/mol).
Moreover, the weighted average mass can be near-integer, but at the same time not corresponding to the mass of any natural isotope. For example, bromine has only two stable isotopes, 79Br and 81Br, naturally present in approximately equal fractions, which leads to the standard atomic mass of bromine close to 80 (79.904 g/mol),[10] even though the isotope 80Br with such mass is unstable.
References[edit]
- ^Jensen, William B. (2005). The Origins of the Symbols A and Z for Atomic Weight and Number. J. Chem. Educ. 82: 1764. link.
- ^'How many protons, electrons and neutrons are in an atom of krypton, carbon, oxygen, neon, silver, gold, etc..?'. Thomas Jefferson National Accelerator Facility. Retrieved 2008-08-27.
- ^'Elemental Notation and Isotopes'. Science Help Online. Archived from the original on 2008-09-13. Retrieved 2008-08-27.
- ^Suchocki, John. Conceptual Chemistry, 2007. Page 119.
- ^Curran, Greg (2004). Homework Helpers. Career Press. pp. 78–79. ISBN1-56414-721-5.
- ^Atoms with the same mass number.
- ^Wang, M.; Audi, G.; Kondev, F. G.; Huang, W. J.; Naimi, S.; Xu, X. (2017). 'The AME2016 atomic mass evaluation (II). Tables, graphs, and references'(PDF). Chinese Physics C. 41 (3): 030003-1–030003-442. doi:10.1088/1674-1137/41/3/030003.
- ^'mass excess, Δ'. International Union of Pure and Applied Chemistry. Retrieved 2021-01-13.
- ^'IUPAC Definition of Relative Atomic Mass'. International Union of Pure and Applied Chemistry. Retrieved 2021-01-13.
- ^'Atomic Weights and Isotopic Compositions for All Elements'. NIST.

Further reading[edit]
- Bishop, Mark. 'The Structure of Matter and Chemical Elements (ch. 3)'. An Introduction to Chemistry. Chiral Publishing. p. 93. ISBN978-0-9778105-4-3. Retrieved 2008-07-08.
- Compassionate Wellness
- Locally owned and operated
If you have an issue with your online order, please contact the store you ordered from by phone - Williamstown 413-458-6244 or Orange 978-633-4225
We are currently operating two adult-use only retail stores to provide wellness and enjoyment through cannabis. Please check our menu for daily updates and sign up for our newsletter to stay up to date on Silver Therapeutics!
Visit us for a Quick, Enjoyable Experience!
We are open 10am to 8pm, 7 days a week for recreational cannabis sales. With less than a 5-minute wait time, our guests are able to enjoy a relaxed shopping experience. We accept cash and debit cards!
Our core values
We exist to provide access to quality cannabis. Our trusted team of cannabis consultants are available at our recreational dispensaries in Williamstown, MA and Orange, MA to help guide your purchasing decisions.

How Many Protons Are In Silver
Community
Compassionate
Silver Atom With A Mass Number Of 109
Integrity
Stay in the loop for store openings, menu offerings and all things Ag! Free download soft for mac.
Your Premier Recreational Dispensaries in Massachusetts and BeyondSilver Therapeutics
Visit one of our locally owned & operated recreational dispensaries in Massachusetts today. Whether you visit us in Orange or Williamstown, you’ll be met with a diverse selection of top-quality cannabis products & a friendly & knowledgeable staff. Yubico ssh. Our Williamstown dispensary is conveniently located near the Eastern New York border, making it a suitable choice for customers who may be visiting us from Saratoga Springs or Albany.
Why Choose Silver Therapeutics Over Other Dispensaries?
When you visit a Silver Therapeutics location, you can be confident in the quality of the cannabis you receive. Whether it’s in the form of a pre-rolled joint, a high potency concentrate, a chocolate or another kind of edible, our cannabis products are consistent and trustworthy.
Our Commitment to Wellness
Silver-108 Mass Number
Silver Therapeutics is committed to providing a unique sense of community, wellness, and quality with every visit. We’re more than a “weed store” or “pot shop” – and we know you’ve got lots of options when it comes to dispensaries in MA. Our staff will answer any question you may have including which product may work best for your unique situation. And better yet, if we don’t know the answer, we are always happy to do our research and get back to you with an informed response.
Ag Atomic Mass
Visit One of Our Several Locations
Number Of Neutrons In Silver
Our Williamstown dispensary is in North Western Mass, just up the street from North Adams and a short drive from Pittsfield, MA. Our Williamstown shop is also incredibly close to Vermont, so we also see visitors from Bennington& Brattleboro. Our dispensary in Orange is only about an hour and twenty minutes East of Williamstown. Silver Therapeutics is expanding! Both of our operating locations in Williamstown and Orange, MA are Adult-Use Retail Stores while at our HCA dispensary in Boston, we can cultivate & process cannabis while selling to both medical AND recreational customers. Our Host Community Agreement (HCA) has been secured for our dispensary location in Roslindale (in Boston) and we will be opening our doors soon!
