- List Of Valence Electrons For Each Element
- Number Of Valence Electrons In Helium Atom
- Total Number Of Valence Electrons In Helium
Electron Configuration
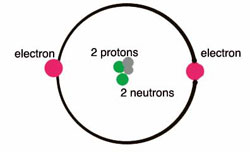
Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas, the first in the noble gas group in the periodic table. For example, in above, Helium (He) and Neon (Ne) have outer valence shells that are completely filled, so neither has a tendency to gain or lose electrons. Therefore, Helium and Neon, two of the so-called Noble gases, exist in free atomic form and do not usually form chemical bonds with other atoms.
Valence Electrons and Ion Formation for the First 20 Elements Element Total Number of Electrons in Neutral Atom Valence Electrons Gain or Lose Electrons Ion Formed Hydrogen 1 1 Gain or Lose 1 H+ or H-Helium 2 2 None None Lithium 3 1 Lose 1 Li+ Beryllium 4 2 Lose 2 Be2+ Boron 5 3 Lose 3 B3+. There are two ways to find the number of valence electrons in Hydrogen (H). The first is to use the Periodic Table to figure out how many electrons Hydrogen.

Python django tutorial. The electrons in an atom fill up its atomic orbitals according to theAufbau Principle; 'Aufbau,' in German, means 'building up.' The AufbauPrinciple prescribes a few simple rules to determine the order atomicorbitals are filled with electrons:
- Electrons always fill orbitals of lower energy first. 1s isfilled before 2s, and 2s before 2p.
- If two electrons occupy the same orbital, they must have opposite spin, as required by the Pauli Exclusion Principle.
- When electrons have to choose between two or more orbitals of thesame energy, electrons prefer to go into different orbitals. As more electrons as added to the atom, these electrons tend to half-fill orbitals of the same energy before pairing with existing electrons to fill orbitals. This isknown as Hund's Rule.
Valency and Valence Electrons
The outermost shell of an atom is its valence shell, and the electrons in the valence shell are valence electrons. Ppg transmission honda. Valence electrons are the highest energy electrons in an atom and are therefore the most reactive. While inner electrons (those not in the valence shell) typically don't participate in chemical bonding and reactions, valence electrons can be gained, lost, or shared to form chemical bonds. For this reason, elements with the same number of valence electrons tend to have similar chemical properties, since they tend to gain, lose, or share valence electrons in the same way. The Periodic Table was designed with this feature in mind. Each element has a number of valence electrons equal to its group number on the Periodic Table.
The electron configurations for the first and second row elements are shownin in simplified notation.
The Octet Rule

Our discussion of valence electron configurations leads us to one of thecardinal tenets of chemical bonding, the octet rule. The octet rulestates that atoms become especially stable when their valence shells gain afull complement of valence electrons. For example, in above, Helium (He) and Neon (Ne) have outer valence shells that are completely filled, so neither has a tendency to gain or lose electrons. Therefore, Helium and Neon, two of the so-called Noble gases, exist in free atomic form and do not usually form chemical bonds with other atoms.
Most elements, however, do not have a full outer shell and are too unstableto exist as free atoms. Instead they seek to fill their outer electronshells by forming chemical bonds with other atoms and thereby attain Noble Gas configuration. An element will tend to take the shortest path to achieving Noble Gas configuration, whether that means gaining or losing one electron. For example, sodium, which has a single electron in its outer 3s orbital, can lose that electron to attain the electron configuration of neon. Chlorine, with seven valence electrons, can gain one electron to attain the configuration of argon. When two different elements have the same electron configuration, they are called isoelectronic.
Valence electrons are those electrons in the outer shell of a given atom, the number of which determines how atoms interact with each other. An atom is said to have a closed shell when it has enough valence electrons in order to make it stable; when there are not enough, it is said to have an open shell. An atom with an open shell is constantly trying to reach stability, forming one of the foundations of many chemical reactions.
An atom is reactive or inert depending on how many valence electrons it has. The most reactive atoms are those that have one or two to lose or those that have one or two to gain in order to maintain stability. For this reason, the noble gases — all of which have a closed outer shell in nature — are chemically inert. In general, eight electrons are needed for an atom to reach stability. Two notable exceptions are hydrogen and helium, both of which need two to make a closed shell.

List Of Valence Electrons For Each Element
The affinity for atoms to achieve stability by gaining or losing valence electrons provides a foundation for two types of chemical bonds: the ionic bond and the covalent bond. Ionic bonds are formed when one atom “steals” an electron from another. Table salt (NaCl) is an example of this. Sodium (Na) has one electron to give up. Chlorine (Cl), on the other hand, needs one to be complete.
To reach stability, chlorine will take an electron from sodium. This allows both elements to achieve a closed shell and stability. The result of this is that the sodium atom becomes a positive ion and the chlorine atom becomes a negative ion. The opposite charges will then attract each other. When in a solution, these molecules also conduct electricity, since ions are free to move around in the solution.
Number Of Valence Electrons In Helium Atom
:max_bytes(150000):strip_icc()/Iodine-58b601a35f9b5860464bfeb4.jpg)
Total Number Of Valence Electrons In Helium
Water is an example of atoms forming a covalent bond. Hydrogen has one atom to gain or lose and oxygen needs two to achieve stability. In this application, however, the oxygen does not steal the electrons from the two hydrogen atoms. Rather, the oxygen and the two hydrogen atoms share the electrons, forming a water molecule. Atoms can also use covalent bonds to share electrons with atoms of the same element, such as in a hydrogen molecule (H2).
