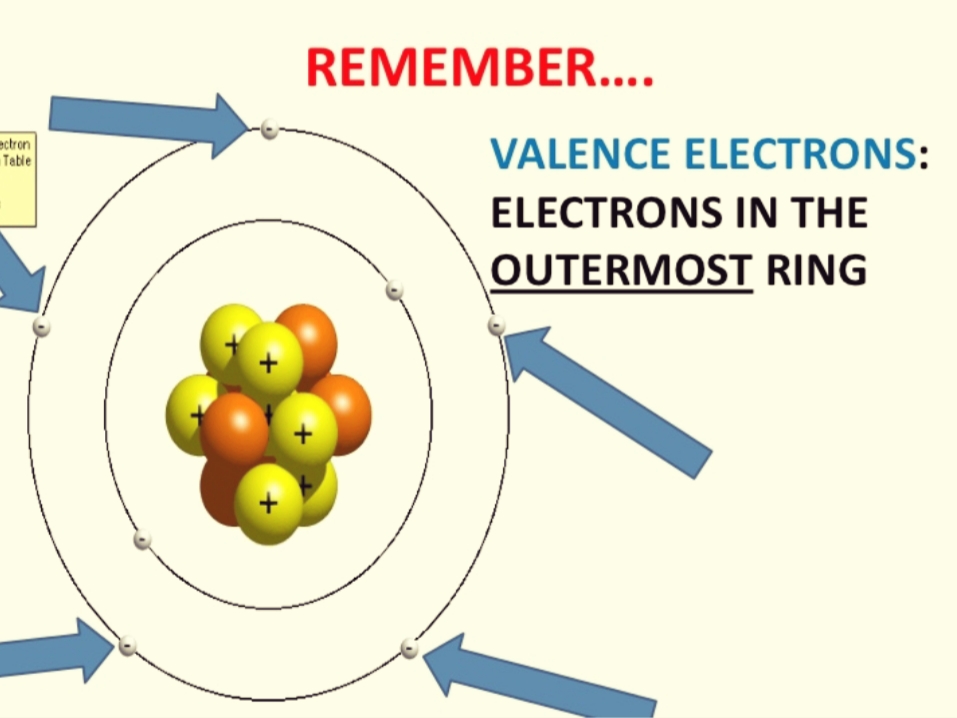
The normal valance number of silicon is 4, so with Si 2- one knows that silicon now has 6 electrons in it's valence shell. Answer: The elements that has four valence electrons are hafnium (Hf) and silicon (Si). Explanation: Electronic configuration: It is defined as the representation of electrons around the nucleus of an atom. Number of electrons in an atom are determined by the electronic configuration. Silicon is element 14 in the Periodic Table It has two electrons in its first shell, eight electrons in the second shell, and four electrons in the third shell. Since the electrons in the third shell are the outermost electrons, silicon has four valence electrons. Calculate the density of valence electrons in silicon. A unit cell of silicon atoms has 8 atoms at corner, 6 atoms at face and 4 atoms within the cell. Number of valence electrons in each atom of Silicon is. Chapter 1, Problem 22 is solved.
Valence-Band/Outer-Shell Electrons
- Practical Electron Microscopy and Database -
- An Online Book -
| https://www.globalsino.com/EM/ |
Based on band theory, when a large number of atoms combine to form a large aggregate, molecular orbitals merge into an almost continuous band of energy levels. The bottom half (called valence band) of the band is composed of bonding M.O.s (molecular orbitals) and is filled with electrons. The upper half (called conduction band) of band is composed of antibonding M.O.s and is empty. In general, those electrons in different orbitals form their own energy levels of valence and conduction bands as shown in Figure 2633a. Figure 2633a. Schematic illustration of evolution of the atomic s and p orbitals into valence and conduction bands in some materials. For instance, the schematic illustration in Figure 2633b shows the bonding levels for diamond and indicates the formation of valence band. When the hybrid carbon atoms bond, a second electron is contributed to the state by the other atom, and thus the interaction between the two electrons lowers the energy of the state. Therefore, the sp3 energy level splits into a series of four bonding and four antibonding orbitals. In an actual solid, there is a Coulomb interaction between the atom cores and the electrons, and then the bonding and anti-bonding energy levels split to form a continuous band structure, with the band of bonding states being the valence band and the anti-bonding states being the conduction band. Figure 2633b. Schematic illustration showing the progression of the electronic structure for an sp3 bonded system. The valence band electrons normally originate from the electrons in the incomplete outer shell of atoms, for instance, the valence band is formed for silicon (Si) crystals as shown in Figure 2633c. An isolated Si atom contains 14 electrons, which occupy 1s, 2s, 2p, 3s and 3p orbital in pairs. When atoms are far away from each other, the electrons in the out shell do not interact. Webrtc firefox. As the distance between atoms is reduced to d1, there is an overlap of electron wavefunctions across adjacent atoms. This leads to a splitting of the energy levels consistent with Pauli exclusion principle, and forming energy bands. This splitting leads to 2N states in the 3s band and 6N states in the 3p band, where N is the number of Si atoms in the crystal. A further reduction of the lattice spacing causes the 3s and 3p energy bands to merge into a single band having 8N available states, and then split again into two bands containing 4N states each. At the temperature of zero Kelvin, the lower band is completely filled with electrons and named as the valence band. The upper band is empty and named as the conduction band. Note that in crystal Si (with lattice spacing d0), the core level electrons do not start yet to interact. Figure 2633c. Detailed illustration of electronic structure of silicon as a function of distance between atoms. The left red circle is a zoom-in of the right red circle. Those valence-band electrons are the only electrons: Figure 2633d shows the schematic illustration of the energy level diagrams for molecular structures with different number of atoms which are single atoms, dimers, clusters and bulk materials. Splitting of the atomic energy levels occurs when the single atoms form a diatomic molecule. As more atoms join the system, the levels split further until a quasi-continuous band structure is formed in the bulk material. In other words, quantum size effects occur when the quasi-continuous band structure of a solid state system begins to break down as more atoms are included. Figure 2633d. Schematic illustration of the energy level diagrams for molecular structures with different number of atoms which are single atoms, dimers, clusters and bulk materials. The gap between the top of valence band and the bottom of conduction band is defined as energy gap Eg. In insulators, the energy gap is very large and no vacant conduction band is available to the valence electrons. Otherwise, the energy gap is either zero due overlapping between valence and conduction bands for metals or is very small for semi-conductors so that conduction band is easily available to valence electrons. For instance, the density (n) of valence electrons in Si3N4 is given by, The sp2 bonded solids have both σ/σ* and π/π* states available to the electrons, while for sp3 bonded solids only the σ/ σ* states present. For the diamond and graphite, electron transitions to these states generate many of the characteristic features in EEL spectra. For instance, in core loss spectra from diamond, the excitation of the 1s electrons to the σ* states generates the carbon k edge peak. For graphite, the transitions of the inner shell electrons into unoccupied π* states give a peak prior to the edge onset. In the valence band, valence electron transitions into the π* states also produce a peak at around 6 eV. |
p-n junction diodes are made up of two adjacent pieces of p-type and n-type semiconducting materials. p-type and n-type materials are simply semiconductors, such as silicon (Si) or germanium (Ge), with atomic impurities; the type of impurity present determines the type of the semiconductor. The process of purposefully adding impurities to materials is called doping; semiconductors with impurities are referred to as 'doped semiconductors'.
p-type


In a pure (intrinsic) Si or Ge semiconductor, each nucleus uses its four valence electrons to form four covalent bonds with its neighbors (see figure below). Each ionic core, consisting of the nucleus and non-valent electrons, has a net charge of +4, and is surrounded by 4 valence electrons. Since there are no excess electrons or holes In this case, the number of electrons and holes present at any given time will always be equal.
An intrinsic semiconductor. Note each +4 ion is surrounded by four electrons.
Now, if one of the atoms in the semiconductor lattice is replaced by an element with three valence electrons, such as a Group 3 element like Boron (B) or Gallium (Ga), the electron-hole balance will be changed. This impurity will only be able to contribute three valence electrons to the lattice, therefore leaving one excess hole (see figure below). Since holes will 'accept' free electrons, a Group 3 impurity is also called an acceptor.
A semiconductor doped with an acceptor. An excess hole is now present.
Because an acceptor donates excess holes, which are considered to be positively charged, a semiconductor that has been doped with an acceptor is called a p-type semiconductor; 'p' stands for positive. Notice that the material as a whole remains electrically neutral. In a p-type semiconductor, current is largely carried by the holes, which outnumber the free electrons. In this case, the holes are the majority carriers, while the electrons are the minority carriers.
n-type
In addition to replacing one of the lattice atoms with a Group 3 atom, we can also replace it by an atom with five valence electrons, such as the Group 5 atoms arsenic (As) or phosphorus (P). In this case, the impurity adds five valence electrons to the lattice where it can only hold four. This means that there is now one excess electron in the lattice (see figure below). Because it donates an electron, a Group 5 impurity is called a donor. Note that the material remains electrically neutral.
A semiconductor doped with a donor. A free electron is now present.
Donor impurities donate negatively charged electrons to the lattice, so a semiconductor that has been doped with a donor is called an n-type semiconductor; 'n' stands for negative. Free electrons outnumber holes in an n-type material, so the electrons are the majority carriers and holes are the minority carriers.
References
Si Valence Electrons Vs
- 'Chapter 6: Diodes.' Fundamentals of Electrical Engineering. 2nd ed. New York, New York: Oxford UP, 1996. 352-54. Print.
